An original paper lost to archives
This paper is an archived paper from the J.Brit.Pod.Med (1996) 51(12):171-174. Apart from its archive benefit, the date of this peer reviewed publication was 1996. From an academic standpoint this forms a useful base for undergraduate scientific writing and critique so I have left the design mistakes rather than re-writing the paper. The figures have been re created from the original article and data. Data included provides a snap shot of the usage at the time in a podiatry clinic supported by local GP services. (Editor-author)
A printable copy is available here.
The use of two injectable corticosteroid preparations used in the management of foot problems – a clinical audit report. David R Tollafield and Helen A Williams
Summary
This audit report attempts to set strict criteria to outcome measurement where two corticosteroids, Methylprednisolone acetate (Depomedrone) and Betamethasone sodium phosphate (Betnesol) were studied prospectively in 66 subjects. While little difference was shown to exist between each drug, Methylprednisolone appeared to have longer lasting activity. Few problems were shown to exist during the study. ‘Steroid flare’ arose in 1.5% of cases and represented the main side effect. Success varied for many common foot conditions treated, both medications fared less well with corticosteroid management at six months or longer. Corticosteroid preparations do offer a useful and worthwhile conservative approach before surgical management is attempted, however, the duration of each condition before primary injection is likely to determine the efficacy of such treatment and will require a separate study. Corticosteroids used in 1992-3 (at the time of study) were administered under GP direction and prescription.
Introduction
There has been no previous podiatric publication regarding the use of local corticosteroids in the UK; indeed, in the USA podiatric literature studies have often been conducted empirically by retrospective analysis. It was decided to look at the range of foot pathology routinely treated with local corticosteroids to establish the clinical effectiveness of two preparations. Local glucocorticoid action is a common method used to treat inflammatory pain and one that is well recognized in clinical practice the world over. The anti-inflammatory effect is achieved via several mechanisms:
- inhibiting chemotactic migration of white blood cells;
- reducing permeability of synovial membrane;
- stabilization of leucocyte lysosomal membranes thus preventing release of enzymes.
The drugs most frequently selected in USA podiatry include Methylprednisolone acetate (Medrol); Betamethasone (Celestone); Triamcinolone acetonide (Kenalog). In the UK licensing of drugs is different and Dexamethasone (Decadron) is used more frequently than Betamethasone (Betnesol – Evans) being used primarily for marked sensitivity reactions and soft tissue, rather than intra articular problems.
Greenfield et al, 1984, reported upon the use of the above drugs with 1% xylocaine for interdigital neuroma. Patients were found to favour injection therapy over the conservative approaches with shoewear style modification or orthotic prescription. In Greenfield’s retrospective study of 65 patients, 50% temporary relief was identified for a mean of 3 injections. Total relief was recorded in 14% of patients after one injection. Injection therapy failed in 11 patients who then went on to require surgical excision; of these cases 4 did poorly. Methylprednisolone acetate (MPA) has approximately 5 times the glucocorticoid action of hydrocortisone, and is some 1.25-1.5 times stronger than prednisolone. Betamethasone is 8-10 times as active as prednisolone on a weight for weight basis.
MPA can be used in all joints of the foot and plantar fascia. The drug is safe but can, in 2% of cases, cause a steroid flare which subsides with the local inflammatory response in 12-48 hours. Table 1 provides a summary of the licensed indications for use of both MPA and Betamethasone in the UK.
| Methylprednisolone | Betamethasone |
| Osteoarthritis
Rheumatoid arthritis Keloid* Granuloma annulare Synovitis* Tenosynovitis Bursitis |
Capsulitis
Heel pain Post operative swelling Status Asthmaticus Tennis Elbow Neuroma Bursitis Acute allergic reactions |
Table 1 Licensed indications and conditions used in this study marked by an asterisk* Drugs used in this study: Methylprednisolone acetate as Depomedrone (Upjohn). Betamethasone sodium phosphate as Betnesol (Evans Medical Ltd)
Contraindications include avoidance in the presence of infection, where patients are immunocompromised or have had any previous allergy to steroids. An allergy which relates to one local steroid does not preclude use of another automatically.
| -2 getting worse |
| -1 steroid flare 1-2 days |
| 0 No change |
| 1 comfortable for 2 weeks |
| 2 comfortable for 2-4 weeks) although still |
| 3 comfortable for 5-8 weeks) some symptoms |
| 4 comfortable for 9-12 weeks |
| 5 comfortable free 3-6 months |
| 6 comfortable free 6 months+ |
Table 2 Scale of clinical changes in symptoms
Much has been recorded on the contraindication of infiltration around tendons. While this does not concern this paper, it should be noted as a cause of premature rupture in tendons such as the tendo Achillis.2.3
The use of methylprednisolone as an intra articular injection has been implicated as having a deleterious effect on cartilage. Hydroxyapatite crystal deposition following steroid injection has been noted in animal research and is thought to increase the effects of osteoarthritis.4 Loss of matrix glycosaminoglycans can lead to replacement with collagen causing a reduction in the flexibility and resilience of cartilage. However, the overall effect on cartilage may not be completely detrimental as decreased swelling protects synovial vessels, stabilizes chondroblasts and intimal cells of the synovium.5 Shoemaker et al (1992) sacrificed horses in a controlled study where carpal joints were deliberately damaged. In those animal injected for four weeks, no replacement fibrocartilage was demonstrated compared with the control group.5
A balance seems to exist between accepting that steroid inflicts cartilage loss with inhibition of repair in favour of improving the synovial production mechanism. Drug specificity may in fact limit damage; shorter acting water soluble sodium phosphate preparations exhibit rapid rates of absorption and shorter duration of action when compared with acetonide esters. The reduction of crystal deposition may limit synovitis. Acetonide esters have an enhanced lipid solubility, prolonged action and slower rate of absorption. Boegel and Miller (1992)6 reinforce the view that when injected in doses of no more than 4mg/ml (1ml) to avoid adversely weakening tissue defenses, Dexamethasone phosphate acts as a short acting soluble corticosteroid diminishing pain and swelling.
Systemic absorption occurs when corticosteroids are injected. The glucocorticoid effects of doses should be appreciated; absorption is affected by drug solubility, dose and surface area. Where two joints are injected with 0.2ml each, the effect upon systemic absorption is greater than when one joint is injected with 0.4ml. In the case of MPA, plasma levels of cortisol have been shown to stay depressed for up to 4 weeks following a single injection. 40-80mg MPA will cause a delayed suppression 2-4 days after treatment.7
Clinical problems with corticosteroid injections do appear rarely. Greenfield et al have recorded ‘steroid flare’.1 Miller indicates that this flare results from the use of less soluble steroid salts when treating neuromata.8 Hopper and Carter (1993) reported anaphylaxis after injecting a knee with MPA and bupivacaine.9 Rapid absorption from the inflamed joint may well have produced toxic levels. No follow-up to isolate the drug responsible leaves this report somewhat unsubstantiated, although the signs of reaction were undoubtedly that of anaphylaxis. Such evidence appears isolated to this single report in recent literature. While the risk of anaphylaxis is potentially possible in a great many drugs, such risks with corticosteroids do appear negligible and relate to local sensitivity alone.
Reactions from such drugs have been examined following intralesional injections. Rasonen and Hasan (1993) looked at MPA and Betamethasone as well as other corticosteroids using 5 known sensitive patients; each produced a delayed rather than anaphylactic reaction.10 Erythema developed on the trunk and face within 3-24 hours, lasting for a short period of 1-3 days. Even in known cases of sensitivity no systemic reaction was provoked. Crystals of steroid may be retained for some months before becoming absorbed. Rest should be advised for 24 hours to prevent crystal synovitis in joints. At local sites, MPA has been shown to create muscle atrophy and lipolysis. Patients undergoing neurectomy at our surgical centre were found to have an organized deposit of MPA within the tissues following small volumes of injection.
Much of the information published to date reveals a general acceptance of corticosteroid injections in medicine. The use of clinical audit applied to a podiatry clinic using injectable corticosteroid was considered important for two reasons: first corticosteroid use has not been reported in UK podiatry, and second because corticosteroids have been considered as a routine podiatric and medical treatment, few studies have looked at the effect on foot conditions critically.
Method
Sixty-six patients were clinically diagnosed as having an inflammatory condition suitable for local corticosteroid infiltration presenting with no previous history of sensitivity or current infection. X-rays were ordered when joints were involved. The frequency of conditions treated is given in Figure 1. Injection therapy was provided in small doses to the affected parts of the foot and details recorded. Post injection follow-up included patient questionnaire. The questionnaire concerned the level of improvement achieved, using a graded score based on duration of symptom relief (Table 2). Analysis of data provides information about the effectiveness of local corticosteroid injection for different conditions and the difference in effect between two selected drugs, Methyl prednisolone (MPA) and Betamethasone sodium phosphate.
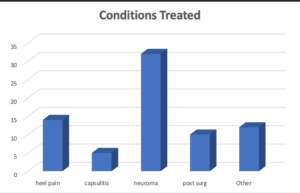
Figure 1 Conditions managed by corticosteroid were grouped as shown to show the frequency of common foot pathology
Results
Sixty-six patients aged between 16-77 were recorded. Two thirds of patients were female. Left and right feet were attended to similarly. Over the study period patients received a total of 126 injections; 22% (n=28) with Betamethasone (Betnesol), 78% (n=98) with MPA (Depomedrone). At any single visit the number of injections for a single patient ranged between 1 and 4, mean = 1.82 injections. The maximum number of injections over the total study period for one patient was 8 infiltrations over 3 visits for several sites. The volume of local corticosteroid administered is shown in Table 3.
Neuromata were frequently treated; the majority of patients were managed with MPA. 54 injections of MPA were used as opposed to 5 injections of Betamethasone. Patients with heel pain were the second largest group treated with 31 injections being provided.
| Range Volume | Mean Volume | Mean Dose Equivalent | |
| 40mg/ml MPA | 0.1 – 0.75 mls | 0.37mls | 14.8mg |
| 4mg/ml Betn | 0.25 -1.0mls | 0.51mls | 2.03mg |
Table 3 Injection administration data
Level of Improvement
Using the rating +1 to +6, i.e slight to full relief of symptoms over all conditions treated, 76% improvement was achieved with MPA as compared to 62% with Betamethasone. Using only score values of +5 and +6, where the patient was still symptom free at 3 months or longer for all conditions treated, 36% improvement was achieved with MPA compared to 15% with Betamethasone (Figure 2). The improvement seen in patients with specific conditions was also noted (Table 4). Capsulitis showed a high rate of symptom reduction over the first three months. No patients appeared to experience problems for longer than this period; one patient was lost to follow up.
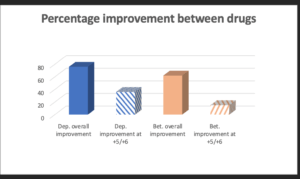
Figure 2 The different effects between methyl prednisolone acetate and betamethasone sodium phosphate for any improvement for all conditions (darker shading), and for strict criteria as graded by +5 or +6 at 3-6 months post injection (light shading) are shown
| Condition | Improvement | Improvement
+5 to +6 |
| Heel pain
Capsulitis Neuroma Post surgery |
64%
75% 75% 40% |
36%
75% 39% 20% |
Table 4 Two measured levels of improvement for both methylprednisolone and betamethasone in a collective patient group of 66 subjects
Discussion
MPA has been one of the principle standard preparations favoured in the literature and is commonly found in general medical practice in the UK. Findings of soft tissue crystallization associated with MPA raised some concern in our study. Another drug, Betamethasone as sodium phosphate (Betnesol) offered a water soluble solution of corticosteroid as an alternative preparation. This same preparation is usually mixed with acetate preparations in the USA.
Majority of conditions fell into the soft tissue category such as Morton’s neuroma, heel pain, fasciitis, plantar bursitis and hypertrophic scars. The use of corticosteroids for post operative pain in the absence of infection was an additional condition not previously recorded in the UK literature. This study found single injections helpful in reducing persistent swelling, particularly following excisional arthroplasties in lesser digits. No delay in healing or any other undesirable sequelae arose in the group of patients who represented post surgical cases.
Lesser MTP joints were predominantly injected during the period of audit for capsulitis/synovitis – pain arising during joint motion. The only other substantial foot joint injected was the subtalar joint in a rheumatoid arthritic patient. In this case the patient was referred by the rheumatology department for injections every six months as an alternative to triple arthrodesis. Pain control was acceptable and provided the patient with substantial although temporary improvement in mobility.
The follow up period varied; the longest period of review reached 52 weeks, the mean period 7.6 weeks. No complications, such as infection, arose in any patient, although one patient was injected at the back of the heel for a suspected neuroma, later identified at surgery. The skin atrophied following a very tender flare up and required excising as part of the neuroma excision. Steroid flare (recorded as -1) was identified in 1.5% of cases and settled within 2-5 days; this figure appears consistent with the literature.1 In the case of neuromata, 27 cases were injected with corticosteroid. 12% required surgery where improvement was not sustained. 25% had no effect, while 39% showed improvement (+5/+6). This value compared favourably with Greenfield et al who found a need to operate on 16% of their patients.1
Loss of data arose in 3 cases. Not all records were completed satisfactorily and data could not always be cross checked despite a follow up questionnaire sent out to patients. The duration of audit might, ideally, have been longer. The length of action of steroid determined by effectiveness varied within this study. The frequency of injections can be repeated from one to five or more weeks for intra-articular injections, but on the basis of evidence already cited, the authors consider that 4-6 weekly is best for soft tissue and joints depending upon the effectiveness of relief. A maximum of 3 injections was deemed optimal for neuroma pain before surgery.
The overall effect of corticosteroid in helping conditions was lower than previously thought being based upon empirical clinical judgement. The low rate of improvement reflected symptom free patients being completely free for >3 months. While improvement was shown in many, the audit set out to establish the effectiveness of corticosteroid therapy using strict criteria, namely reaching a score of +5 or +6. Miller et al (1995) looked at the effects of first time corticosteroid injections for heel syndromes (n=24 cases) using Betamethasone.11 Only single injections were undertaken. While the short term results were encouraging, the maximum benefit lasted only 6-8 weeks. At final follow up of at least five months, only 41% had a satisfactory result based on a subjective pain relief grading of excellent, good, fair or poor. The use of multiple injections is likely to yield better results.
Betamethasone appeared to be less effective than MPA. In particular longer effects sustained by MPA may have been more advantageous. Some caution is given to possible interpretation of the difference between the corticosteroids used in this report. The number of MPA injections used related to a much larger group of patients (n=82) while Betamethasone sodium sulphate (n=17) accounted for only some 20% of patient injections. The success attributed to MPA predominated in neuroma injections of which 54 were given to patients as opposed to only 5 using Betamethasone.
Conclusion
While problems have been recorded in the literature they are usually associated with delayed reactions. The volumes likely to be used in the foot should follow the drug information for small joints and soft tissues; excessive volumes of >30mg of MPA have been less well tolerated than Betamethasone and are most likely associated with its solubility. The authors do not consider that this study provides sufficient evidence to support the use of MPA over Betamethasone, except that MPA does appear to last longer, a point consistent with literature.
The report used strict criteria to evaluate the effect of corticosteroid. In many cases the problem had been present for periods of up to two years. The efficacy of corticosteroid is likely to be affected by conditions of longer duration. Future studies need to consider normalizing such data to avoid confounding factors.
This study confirms that corticosteroids have a useful part to play in foot management in the hands of trained podiatrists and that, by following drug guidelines, side effects associated with such injections are predictable and safe for use within the clinical environment. Only 11% of GP’s (n=112) wanted to undertake steroid injections, most preferring podiatrists to undertake the technique.12 Suitable emergency drugs for resuscitation purpose should be made available, however, and defibrillation equipment regularly serviced.
The use of audit tools is essential for evaluating treatment. Clinicians should evaluate all forms of management to ensure that acceptable practices are followed, ideally by prospective analysis. While scientific methodology is preferred, the difficulties associated with collecting data in a busy multipurpose clinic can create problems in reviewing patients at correct end points in studies of this nature.
Acknowledgements
Data in the paper was collected from two centres: Northampton School of Podiatry Clinic (now University of Northampton) and Walsall Community Health. Both names have changed and WCH was amalgamated within the Acute Trust during 2011.
Editor’s note: This original paper was written before podiatrists had legal access to steroids.The drugs were not administered under GP direction although the acquisition of the drug was provided by each patient’s own GP. Steroids were not available to podiatrists until 2006. In the last 26 years progress in writing and research into podiatry has changed exponentially but this paper represents a time when clinical research was not being undertaken into medication and injectable therapy. As universities have implemented degrees at Bachelor and Masters level, dilution of clinical research has sadly arisen due to stricter terms of ethical approval, lack of time and resources. The numbers allocated to studies is often low in clinical research. The ideals of research today include blind patient selection and controls. Other articles by the author-editor can be found on this site – HERE
![]()
- Greenfield J, Rea, J, Ilfield FW. Morton’s Interdigital neuroma: indications for treatment by local injections versus surgery. Clin Orthop Rel Res. Pp142-144, 1984
- Read, TF, Motto SG Tendo achilles pain: steroid and outcome. Br. J Sp Med 26,1 pp15-17, 1992
- Mahler, F, Fritsky, D. Partial and complete ruptures of the achilles tendon and local corticosteroid injections. Br. J Sp Med 26,1 pp7-13, 1992
- Ohira T, Isikawa K, Kumato, S. Hydroxyapatite deposition in articular cartilage by intraarticular injections of methylprednisolone. J Bone & Joint Surg. 68-A, 4:509-510. 1986
- Shoemaker RS, Bertone AL, Martin GS, McIlwraith, CW, Roberts, ED, Pechman, R, Kearney MT. Effects of intraarticular administration of methylprednisolone acetate on normal articular cartilage and on healing of experimentally induced osteochondral defects in horses. Am. J. Vet. Res. 53,8, pp1446-1453, 1992
- Boegel, W, Miller S. Perioperative considerations for foot and ankle surgery. In McGlamry ED, Banks AS, Downey MS eds. Comprehensive textbook of foot surgery. 2nd Ed. P214. Baltimore: Williams and Wilkins 1992
- Pharmaceutical information Evans (Betnesol)
- Miller S. Morton’s Neuroma. A syndrome. In McGlamry ED, Banks AS, Downey MS eds. Comprehensive textbook of foot surgery. 2nd Ed. Pp310. Baltimore: Williams and Wilkins 1992
- Hopper, JM, Carter SR. Anaphylaxis after intra-articular injection of bupivacaine and methylprednisolone. . J Bone & Joint Surg. 75-B pp505-507, 1993
- Rasanen L, Hasan T Allergy to systemic and intralesional corticosteroids. Br.J Dermat. 128, pp407-411,1993
- Miller R, Torres J, McGuire M. Efficacy of first time steroid injection for painful heel syndrome. Foot and Ankle International. 16,10 pp610-612, 1995
- Tollafield DR, Parmar D. Setting standards for day care foot surgery. A quinquennial review. J.Br Podiatr Med. & Surg. 6.1pp7-20,1994 (republished by the Society of Chiropodists & Podiatrists
- Tollafield DR The Dawning of the PGD Reflective Podiatric Practice. BPCC Ltd 2018. 1(6):1-6
Sign-up for here my regular FREE newsfeed
Thanks for reading ‘First use of corticosteroids by UK podiatrists’.
Published by Busypencilcase Communications. Est. 2015

Originally published 1996, and then for consultingfootpain in 2018 reflective podiatric practice.
Open access distribution February 2022.



This is a landmark study. There remains disappointingly little data on the use of cortisone in foot pain, yet as podiatrists we can easily collect large numbers of patients and measure outcomes with validated tools. This is a real opportunity for Masters students in particular. This paper being the first should be an essential reference for all future studies.